Authors
He Fu, Beatrice Desvergne, Serge Ferrari, Nicolas Bonnet
Lab
Geneva University Hospital, and Faculty of Medicine, Geneva, Switzerland
Journal
Endocrinology
Abstract
Fragility fractures are recognized complication of diabetes, but yet the underlying mechanisms remain poorly understood. This is particularly pronounced in type 2 diabetes in which the propensity to fall is increased but bone mass is not necessarily low. Thus, whether factors implicated in the development of insulin resistance and diabetes directly impact on the musculoskeletal system remains to be investigated. PPAR?(-/-) mice have reduced metabolic activity and are glucose intolerant. We examined changes in bone and muscle in PPAR?(-/-) mice and investigated both the mechanism behind those changes with age as well as their response to exercise. Compared with their wild type, PPAR?(-/-) mice had an accelerated and parallel decline in both muscle and bone strength with age. These changes were accompanied by increased myostatin expression, low bone formation, and increased resorption. In addition, mesenchymal cells from PPAR?(-/-) had a reduced proliferation capacity and appeared to differentiate into more of an adipogenic phenotype. Concomitantly we observed an increased expression of PPAR?, characteristic of adipocytes. The anabolic responses of muscle and bone to exercise were also diminished in PPAR?(-/-) mice. The periosteal bone formation response to direct bone compression was, however, maintained, indicating that PPAR? controls periosteal bone formation through muscle contraction and/or metabolism. Taken together, these data indicate that PPAR? deficiency leads to glucose intolerance, decreased muscle function, and reduced bone strength. On a molecular level, PPAR? appears to regulate myostatin and PPAR? expression in muscle and bone, thereby providing potential new targets to reverse bone fragility in patients with metabolic disturbances.
BIOSEB Instruments Used
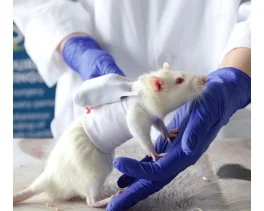
Grip strength test (BIO-GS3)
Keywords/Topics
Diabète, Insuline & Glucose; Vieillissement; Métabolisme; Thématiques transversales
Source :

 Douleur - Allodynie/Hyperalgésie Thermique
Douleur - Allodynie/Hyperalgésie Thermique Douleur - Spontanée - Déficit de Posture
Douleur - Spontanée - Déficit de Posture Douleur - Allodynie/Hyperalgésie Mécanique
Douleur - Allodynie/Hyperalgésie Mécanique Apprentissage/Mémoire - Attention - Addiction
Apprentissage/Mémoire - Attention - Addiction Physiologie & Recherche Respiratoire
Physiologie & Recherche Respiratoire


































 Douleur
Douleur Système Nerveux Central (SNC)
Système Nerveux Central (SNC)  Neurodégénérescence
Neurodégénérescence Système sensoriel
Système sensoriel Système moteur
Système moteur Troubles de l'humeur
Troubles de l'humeur Autres pathologies
Autres pathologies Système musculaire
Système musculaire Articulations
Articulations Métabolisme
Métabolisme Thématiques transversales
Thématiques transversales Congrès & Meetings
Congrès & Meetings 