Authors
W Dong, L Zhang, C Sun et al
Lab
Key Laboratory of Human Disease Comparative Medicine, National Health Commission of China, Institute of Laboratory Animal Science, Peking Union Medicine College, Chinese Academy of Medical Sciences, Beijing, China.
Journal
Animal Models and Experimental Medicine
Abstract
Background: The GGGGCC (G4C2) repeat expansion in the human open reading frame 72 on chromosome 9, C9orf72, is the most common cause of amyotrophic lat- eral sclerosis (ALS). Studies in transgenic mouse models have linked the pathogenic mechanism of G4C2 repeat expansion to RNA foci or the accumulation of unnatural dipeptide repeats in neurons. However, only one of the existing transgenic mouse lines developed typical ALS.
Methods: C9orf72 knockin rats were generated by knockin of 80 G4C2 repeats with human flanking fragments within exon1a and exon1b at the rat C9orf72 locus. Protein expression was detected by western blot. Motor coordination and grip force were measured using a Rotarod test and a grip strength test. Neurodegeneration was as- sessed by Nissl staining with cresyl violet.
Results: C9orf72 haploinsufficiency reduced C9orf72 protein expression 40% in the cerebrum, cerebellum and spinal cords from knockin rats (P < .05). The knockin (KI) rats developed motor deficits from 4 months of age. Their falling latencies and grip force were decreased by 67% (P < .01) and 44% (P < .01), respectively, at 12 months of age compared to wild-type (WT) mice. The knockin of the hexanucleotide repeat expansion (HRE) caused a 47% loss of motor neurons in the spinal cord (P < .001) and 25% (5/20) of female KI rats developed hind limb paralysis at 13 to 24 months.
Conclusion: Motor defects in KI rats may result from neurotoxicity caused by HRE and the resulting reduction in C9orf72 protein due to haploinsufficiency. These KI rats could be a useful model for investigating the contributions of loss-of-function to neurotoxicity in C9orf72-related ALS.
BIOSEB Instruments Used
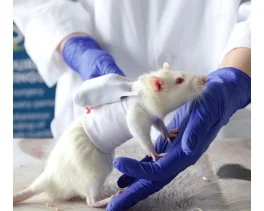
Grip strength test (BIO-GS3)
Source :

 Douleur - Allodynie/Hyperalgésie Thermique
Douleur - Allodynie/Hyperalgésie Thermique Douleur - Spontanée - Déficit de Posture
Douleur - Spontanée - Déficit de Posture Douleur - Allodynie/Hyperalgésie Mécanique
Douleur - Allodynie/Hyperalgésie Mécanique Apprentissage/Mémoire - Attention - Addiction
Apprentissage/Mémoire - Attention - Addiction Physiologie & Recherche Respiratoire
Physiologie & Recherche Respiratoire


































 Douleur
Douleur Système Nerveux Central (SNC)
Système Nerveux Central (SNC)  Neurodégénérescence
Neurodégénérescence Système sensoriel
Système sensoriel Système moteur
Système moteur Troubles de l'humeur
Troubles de l'humeur Autres pathologies
Autres pathologies Système musculaire
Système musculaire Articulations
Articulations Métabolisme
Métabolisme Thématiques transversales
Thématiques transversales Congrès & Meetings
Congrès & Meetings 