Authors
Czopek A, Ko?aczkowski M, Bucki A, Byrtus H et al.
Lab
Department of Pharmaceutical Chemistry, Jagiellonian University Medical College, Kraków, Poland
Journal
Bioorg Med Chem.
Abstract
A series of novel spirohydantoin derivatives with arylpiperazinylbutyl moiety were synthesized and evaluated for serotonin 5-HT1A, 5-HT2A, 5-HT7 and dopamine D2 receptors. Based on these data, four compounds were selected for further binding affinity assays on dopamine D1, D3, D4, and 5-HT2C, 5-HT6 as well as adrenergic ?1 and ?2C receptors, which are involved in various CNS diseases such as schizophrenia, anxiety and/or depression. The compound 14, 1-{4-[4-(2-metoxyphe-nyl)piperazin-1-yl]butyl}-3,4-dihydro-2H,2H,5H-spiro[imidazolidine-4,1-naphthalene]-2,5-dione, with the most promising functional profile, mixed 5-HT2A/D2 antagonist and 5-HT1A partial agonist, was selected. In the mouse d-amphetamine-induced locomotor hyperactivity model, compound 14 produced antipsychotic-like activity, which is devoid of cataleptogenic effects and in the forced swim test in mice, it showed a significant antidepressant-like effect unlike the reference drug aripiprazole.
BIOSEB Instruments Used
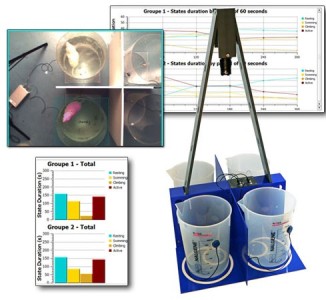
Forced Swimming Test: New FST DUAL SENSOR (BIO-FST-DSM)
Source :

 Douleur - Allodynie/Hyperalgésie Thermique
Douleur - Allodynie/Hyperalgésie Thermique Douleur - Spontanée - Déficit de Posture
Douleur - Spontanée - Déficit de Posture Douleur - Allodynie/Hyperalgésie Mécanique
Douleur - Allodynie/Hyperalgésie Mécanique Apprentissage/Mémoire - Attention - Addiction
Apprentissage/Mémoire - Attention - Addiction Physiologie & Recherche Respiratoire
Physiologie & Recherche Respiratoire


































 Douleur
Douleur Système Nerveux Central (SNC)
Système Nerveux Central (SNC)  Neurodégénérescence
Neurodégénérescence Système sensoriel
Système sensoriel Système moteur
Système moteur Troubles de l'humeur
Troubles de l'humeur Autres pathologies
Autres pathologies Système musculaire
Système musculaire Articulations
Articulations Métabolisme
Métabolisme Thématiques transversales
Thématiques transversales Congrès & Meetings
Congrès & Meetings