L'équipe de Bioseb est fière et heureuse de vous présenter
les derniers travaux de recherche de Transpharmation et The Royal Veterinary College, Londres, Royaume-Uni, de Ran Magnusdottir, Amy Fisher et Neil Upton, qui ont utilisé
la plaque Froid+Chaud de Bioseb dans le cadre de leurs recherches:
Inversion de l'allodynie froide et mécanique grâce à la prégabaline
dans un modèle murin des neuropathies périphériques chimio-induites
Contexte: Les neuropathies périphériques chimio-induites (NPCI) constituent un effet secondaire limitant les doses utilisables de l'oxaliplatine antinéoplastique, basée sur le platine. Il n'existe actuellement aucun traitement pour inverser la neurotoxicité qui se caractérise par des douleurs, une perte sensorielle et une allodynie froide chez jusqu'à 80% des patients.
 Cliquez ici
Cliquez ici
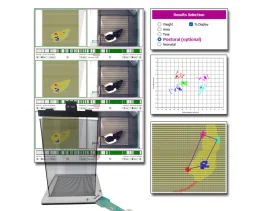
pour voir les courbes Résultats: Des NPCI ont été induites chez 10 souris mâles C57BL/6 (6 semaines) à l'aide d'une injection intra-péritonale unique d'oxaliplatine (15mg/kg i.p.). Des signes d'allodynie froide et mécanique ont été mesurés grâce à
la plaque Froid/Chaud de Bioseb (Bioseb, France) à 20°C, ainsi que des filaments de Von Frey en utilisation manuelle, de la baseline à 20 jours après l'injection. Une allodynie froide et mécanique a été établie 3 jours après l'injection d'oxaliplatine, et est restée stable durant 14 jours. Au quinzième jour, une dose de prégabaline (3mg/kg p.o.) a ramené l'allodynie mécanique à la baseline en deux heures après l'injection, et l'allodynie froide en 1 heure et 2 heures après injection. Après une période de wash-out de 2 jours où les scores d'allodynie sont retournés à leur niveau neuropathique précédent, une nouvelle dose de prégabaline (10 mg/kg p.o.) a ramené les scores de l'allodynie mécanique et froide à baseline en respectivement 1 et 2 heures. Les tests de sensibilité au froid ont été effectués soit directement après le test de Von Frey, soit sans ces derniers, avec les mêmes effets - aucun effet iatrogénique du test de Von Frey n'a ainsi été constaté sur la sensibilité au froid. L'analyse de corrélation des réponses aux stimulis froids et mécaniques n'a pas montré de tendance claire, indiquant que les neuropathies périphériques chimio-induites par l'oxiplatine affectent ces deux modalités de manière différente.
Conclusions: Les neuropathies périphériques chimio-induites (NPCI) par l'oxaliplatine, mesurées via l'allodynie froide et/ou mécanique, sont réversibles via l'emploi d'une simple dose de prégabaline.
Pour plus d'informations, merci de contacter
Amy S. Fisher, PhD - Scientifique principale, Transpharmation Ltd
amy.fisher@transpharmation.co.uk - Tél: +44 (0)17 0764 2162
Web: www.transpharmation.co.uk
La Plaque Froid/Chaud de Bioseb devient un instrument incontournable pour l'étude des allodynies et hyperalgésies froides chez le rongeur. En se concentrant sur l'hypersensibilité thermale induite par l'oxaliplatine, la validité de notre instrument comme test comportemental a été démontrée dans de nombreuses publications, comme par exemple :
• Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. (2011) Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors.
J. Descoeur, V. Pereira, A. Pizzoccaro, A. Francois, B. Ling et al. (Team of Dr Bourinet)
Institut de Génomique Fonctionnelle, CNRS, UMR-5203, Département de Physiologie, Montpellier, France.
Published in "EMBO Molecular Medicine" (2011-05-24)
Cold hypersensitivity is the hallmark of oxaliplatin-induced neuropathy, which develops in nearly all patients under this chemotherapy. To date, pain management strategies have failed to alleviate these symptoms, hence development of adapted analgesics is needed. Here, we report that oxaliplatin exaggerates cold perception in mice as well as in patients. These symptoms are mediated by primary afferent sensory neurons expressing the thermoreceptor TRPM8. Mechanistically, oxaliplatin promotes over-excitability by drastically lowering the expression of distinct potassium channels (TREK1, TRAAK) and by increasing the expression of pro-excitatory channels such as the hyperpolarization-activated channels (HCNs). These findings are corroborated by the analysis of TREK1-TRAAK null mice and use of the specific HCN inhibitor ivabradine, which abolishes the oxaliplatin-induced cold hypersensibility. These results suggest that oxaliplatin exacerbates cold perception by modulating the transcription of distinct ionic conductances that together shape sensory neuron responses to cold. The translational and clinical implication of these findings would be that ivabradine may represent a tailored treatment for oxaliplatin-induced neuropathy.
•
Anticancer Activity of Methyl-substituted Oxaliplatin Analog. (2012) Anticancer Activity of Methyl-substituted Oxaliplatin Analog.
U. Jungwirth, D. Xanthos, J. Gojo, A. Bytzek, W. Korner et al.
Medical University of Vienna, Institute of Cancer Research, Vienna, Austria.
Published in "Molecular pharmacology" (2012-05-30)
Oxaliplatin is successfully used in systemic cancer therapy. However, resistance development and severe adverse effects are limiting factors for curative cancer treatment with oxaliplatin. The purpose of this study was to comparatively investigate in vitro and in vivo anticancer properties as well as the adverse effects of two methyl-substituted enantiomerically pure oxaliplatin analogs [[(1R,2R,4R)-4-methyl-1,2-cyclohexanediamine] oxalatoplatinum(II) (KP1537), and [(1R,2R,4S)-4-methyl-1,2-cyclohexanediamine]oxalatoplatinum(II) (KP1691)] and to evaluate the impact of stereoisomerism. Although the novel oxaliplatin analogs demonstrated in multiple aspects activities comparable with those of the parental compound, several key differences were discovered. The analogs were characterized by reduced vulnerability to resistance mechanisms such as p53 mutations, reduced dependence on immunogenic cell death induction, and distinctly attenuated adverse effects including weight loss and cold hyperalgesia. Stereoisomerism of the substituted methyl group had a complex and in some aspects even contradictory impact on drug accumulation and anticancer activity both in vitro and in vivo. To summarize, methyl-substituted oxaliplatin analogs harbor improved therapeutic characteristics including significantly reduced adverse effects. Hence, they might be promising metal-based anticancer drug candidates for further (pre)clinical evaluation.
•
P2X7 Cell Death Receptor Activation and Mitochondrial Impairment in Oxaliplatin-Induced Apoptosis and Neuronal Injury: Cellular Mechanisms and In Vivo Approach (2013) P2X7 Cell Death Receptor Activation and Mitochondrial Impairment in Oxaliplatin-Induced Apoptosis and Neuronal Injury: Cellular Mechanisms and In Vivo Approach
F. Massicot, G. Hache, L. David, D. Chen, C. Leuxe et al.
Faculté des Sciences Pharmaceutiques et Biologiques, Université Paris Descartes, Paris, France
Published in "PLOS One" (2013-06-27)
Limited information is available regarding the cellular mechanisms of oxaliplatin-induced painful neuropathy during exposure of patients to this drug. We therefore determined oxidative stress in cultured cells and evaluated its occurrence in C57BL/6 mice. Using both cultured neuroblastoma (SH-SY5Y) and macrophage (RAW 264.7) cell lines and also brain tissues of oxaliplatin-treated mice, we investigated whether oxaliplatin (OXA) induces oxidative stress and apoptosis. Cultured cells were treated with 2–200 µM OXA for 24 h. The effects of pharmacological inhibitors of oxidative stress or inflammation (N-acetyl cysteine, ibuprofen, acetaminophen) were also tested. Inhibitors were added 30 min before OXA treatment and then in combination with OXA for 24 h. In SH-SY5Y cells, OXA caused a significant dose-dependent decrease in viability, a large increase in ROS and NO production, lipid peroxidation and mitochondrial impairment as assessed by a drop in mitochondrial membrane potential, which are deleterious for the cell. An increase in levels of negatively charged phospholipids such as cardiolipin but also phosphatidylserine and phosphatidylinositol, was also observed. Additionally, OXA caused concentration-dependent P2X7 receptor activation, increased chromatin condensation and caspase-3 activation associated with TNF-α and IL-6 release. The majority of these toxic effects were equally observed in Raw 264.7 which also presented high levels of PGE2. Pretreatment of SH-SY5Y cells with pharmacological inhibitors significantly reduced or blocked all the neurotoxic OXA effects. In OXA-treated mice (28 mg/kg cumulated dose) significant cold hyperalgesia and oxidative stress in the tested brain areas were shown. Our study suggests that targeting P2X7 receptor activation and mitochondrial impairment might be a potential therapeutic strategy against OXA-induced neuropathic pain.
•
The Nav1. 9 Channel Is a Key Determinant of Cold Pain Sensation and Cold Allodynia (2015) The Nav1. 9 Channel Is a Key Determinant of Cold Pain Sensation and Cold Allodynia
Lolignier S, Bonnet C, Gaudioso C, Noël J, Ruel J et al.
Pharmacologie Fondamentale et Clinique de la Douleur, Clermont Université, Université d'Auvergne, Clermont-Ferrand, France;
Published in "Cell Rep. " (2015-05-19)
Cold-triggered pain is essential to avoid prolonged exposure to harmfully low temperatures. However, the molecular basis of noxious cold sensing in mammals is still not completely understood. Here, we show that the voltage-gated Nav1.9 sodium channel is important for the perception of pain in response to noxious cold. Nav1.9 activity is upregulated in a subpopulation of damage-sensing sensory neurons responding to cooling, which allows the channel to amplify subthreshold depolarizations generated by the activation of cold transducers. Consequently, cold-triggered firing is impaired in Nav1.9(-/-) neurons, and Nav1.9 null mice and knockdown rats show increased cold pain thresholds. Disrupting Nav1.9 expression in rodents also alleviates cold pain hypersensitivity induced by the antineoplastic agent oxaliplatin. We conclude that Nav1.9 acts as a subthreshold amplifier in cold-sensitive nociceptive neurons and is required for the perception of cold pain under normal and pathological conditions.
•
A Polyamine-Deficient Diet Prevents Oxaliplatin-Induced Acute Cold and Mechanical Hypersensitivity in Rats (2013) A Polyamine-Deficient Diet Prevents Oxaliplatin-Induced Acute Cold and Mechanical Hypersensitivity in Rats
J.Ferrier, M.Bayet-Robert, B.Pereira, L.Daulhac
FACULTE DE MEDECINE, CLERMONT FERRAND, FRANCE
Published in "PLOS ONE" (2013-11-30)
Background
Oxaliplatin is an anticancer drug used for the treatment of advanced colorectal cancer, but it can also cause painful peripheral neuropathies. The pathophysiology of these neuropathies has not been yet fully elucidated, but may involve spinal N-methyl-D-aspartate (NMDA) receptors, particularly the NR2B subunit. As polyamines are positive modulators of NMDA-NR2B receptors and mainly originate from dietary intake, the modulation of polyamines intake could represent an interesting way to prevent/modulate neuropathic pain symptoms by opposing glutamate neurotransmission.
Methods
The effect of a polyamine deficient diet was investigated in an animal model of oxaliplatin-induced acute pain hypersensitivity using behavioral tests (mechanical and cold hypersensitivity). The involvement of spinal glutamate neurotransmission was monitored by using a proton nuclear magnetic resonance spectroscopy based metabolomic approach and by assessing the expression and phosphorylation of the NR2B subunit of the NMDA receptor.
Results
A 7-day polyamine deficient diet totally prevented oxaliplatin-induced acute cold hypersensitivity and mechanical allodynia. Oxaliplatin-induced pain hypersensitivity was not associated with an increase in NR2B subunit expression or phosphorylation, but with an increase of glutamate level in the spinal dorsal horn which was completely prevented by a polyamine deficient diet. As a validation that the oxaliplatin-induced hypersensitivity could be due to an increased activity of the spinal glutamate system, an intrathecal administration of the specific NR2B antagonist, ifenprodil, totally reversed oxaliplatin-induced mechanical and cold hypersensitivity.
Conclusion
A polyamine deficient diet could represent a promising and valuable nutritional therapy to prevent oxaliplatin-induced acute pain hypersensitivity.
•
Assessment of thermal sensitivity in rats using the thermal place preference test: description and application in the study of oxaliplatin-induced acute thermal hypersensitivity and inflammatory pain models. (2014) Assessment of thermal sensitivity in rats using the thermal place preference test: description and application in the study of oxaliplatin-induced acute thermal hypersensitivity and inflammatory pain models.
Balayssac D, Ling B, Ferrier J, Pereira B, Eschalier A, Authier N.
Faculties of Medicine and Pharmacy, Clermont Université, France
Published in "Behav Pharmacol." (2014-04-25)
Thermal sensitivity is an essential characteristic of some painful states, including oxaliplatin-induced neuropathy. The thermal place preference test (TPPT) was designed to finely assess thermal sensitivity in rodents. The TPPT monitors the time spent by unrestrained rodents on a test plate at fixed temperatures (5-50°C) compared with an adjacent reference plate at a neutral temperature (25°C). Here, we report the results of a study designed (i) to validate the optimal methodological parameters for measuring thermal sensitivity in rats, (ii) to assess the thermal sensitivity of healthy rats and animal models of pain and (iii) to explore the pharmacological effects of analgesic drugs. The most reproducible conditions occurred when the TPPT was performed in the morning and in the dark for 3 min with the reference plate set to 25°C. The temperature preferences of healthy rats were more than 17°C and less than 40°C. When compared with control animals, oxaliplatin-treated rats showed thermal hypersensitivity at 12, 20 and 35°C, and carrageenan-treated rats showed thermal hypersensitivity at 15 and 45°C. Duloxetine (2.5 mg/kg, intraperitoneal) reversed oxaliplatin-induced cold hypersensitivity (20°C) and morphine (1 mg/kg, intravenous) reversed carrageenan-induced heat hypersensitivity (45°C). We conclude that the TPPT enables a fine-grained assessment of thermal sensitivity that is relevant to the pathophysiological exploration of animal pain models and to the pharmacological assessment of analgesic drugs.

 Douleur - Allodynie/Hyperalgésie Thermique
Douleur - Allodynie/Hyperalgésie Thermique Douleur - Spontanée - Déficit de Posture
Douleur - Spontanée - Déficit de Posture Douleur - Allodynie/Hyperalgésie Mécanique
Douleur - Allodynie/Hyperalgésie Mécanique Apprentissage/Mémoire - Attention - Addiction
Apprentissage/Mémoire - Attention - Addiction Physiologie & Recherche Respiratoire
Physiologie & Recherche Respiratoire


































 Douleur
Douleur Système Nerveux Central (SNC)
Système Nerveux Central (SNC)  Neurodégénérescence
Neurodégénérescence Système sensoriel
Système sensoriel Système moteur
Système moteur Troubles de l'humeur
Troubles de l'humeur Autres pathologies
Autres pathologies Système musculaire
Système musculaire Articulations
Articulations Métabolisme
Métabolisme Thématiques transversales
Thématiques transversales Congrès & Meetings
Congrès & Meetings 