Authors
Natalie A Duggett, Sarah J L Flatters
Lab
Wolfson Centre for Age-Related Diseases, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, UK
Journal
British Journal of Pharmacology
Abstract
Background and Purpose
Bortezomib (Velcade®) is a breakthrough treatment for multiple myeloma, significantly improving patient survival. However, its use is limited by painful neuropathy often resulting in dose reduction/cessation of first-line treatment due to lack of treatment. The aim of this study was to characterize a clinically relevant rat model of bortezomib-induced painful neuropathy, using established evoked measures and novel ethological techniques, to aid drug discovery.
Experimental Approach
Adult male Sprague–Dawley rats were injected i.p. with 0.1 and 0.2 mg·kg−1 bortezomib, or its vehicle, on days 0, 3, 7 and 10. Multiple behavioural approaches were utilized: mechanical hypersensitivity, cold allodynia, heat hypersensitivity, motor co-ordination, burrowing and voluntary wheel running. At maximal bortezomib-induced mechanical hypersensitivity, 200 mg·kg−1 ethosuximide/vehicle and 100 mg·kg−1 phenyl N-tert-butylnitrone (PBN)/vehicle were administered i.p. in separate experiments, and mechanical hypersensitivity assessed 1, 3 and 24 h later.
Key Results
Bortezomib induced dose-related mechanical hypersensitivity for up to 80 days. Bortezomib induced short-term cold allodynia, but no significant change in heat hypersensitivity, motor co-ordination, voluntary wheel running and burrowing behaviour compared to vehicle-treated controls. Systemic PBN and ethosuximide significantly ameliorated bortezomib-induced mechanical hypersensitivity.
Conclusions and Implications
These data characterize a reproducible rat model of clinical-grade bortezomib-induced neuropathy demonstrating long-lasting pain behaviours to evoked stimuli. Inhibition by ethosuximide and PBN suggests involvement of calcium and/or ROS in bortezomib-induced painful neuropathy. These drugs could be used as preclinical positive controls to assess novel analgesics. As ethosuximide is widely used clinically, translation to the clinic to treat bortezomib-induced painful neuropathy may be possible.
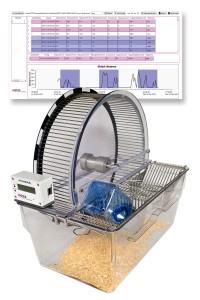
BIOSEB Instruments Used:
Basic spontaneous activity wheels (BIO-ACTIVW-M),Premium spontaneous activity wheels (BIO-ACTIWH2)
Keywords/Topics
Pain; Neuropathic pain

 Pain - Thermal Allodynia / Hyperalgesia
Pain - Thermal Allodynia / Hyperalgesia Pain - Spontaneous Pain - Postural Deficit
Pain - Spontaneous Pain - Postural Deficit Pain - Mechanical Allodynia / Hyperalgesia
Pain - Mechanical Allodynia / Hyperalgesia Learning/Memory - Attention - Addiction
Learning/Memory - Attention - Addiction Physiology & Respiratory Research
Physiology & Respiratory Research











![Dynamic Weight Bearing 2.0 – Postural Module [Add-on]](https://bioseb.com/733-home_default/dynamic-weight-bearing-20-add-on-postural-module.jpg)























 Pain
Pain Central Nervous System (CNS)
Central Nervous System (CNS) Neurodegeneration
Neurodegeneration Sensory system
Sensory system Motor control
Motor control Mood Disorders
Mood Disorders Other disorders
Other disorders Muscular system
Muscular system Joints
Joints Metabolism
Metabolism Cross-disciplinary subjects
Cross-disciplinary subjects CONFERENCES & MEETINGS
CONFERENCES & MEETINGS